Monday April 22, 2024 Day 68 Types of Nuclear Decay and Nuclear Equations |
|||
| Course Lectures Please refer to the daily YouTube videos below |
Textbook References 20.2: The Discovery of Radioactivity 20.3: Types of Radioactivity |
||
Textbook: 21.2 Nuclear Equations 21.6 Biological effects of radiation Radioactivity Objectives: 1. Determine protons, neutrons and electrons given a nuclide symbol. 2. List the types of nuclear radiation, their characteristics and penatrating abilities. 3. Given the identity of the decaying nuclide and it's daughter-product, identify the type of radioactive decay taking place. 4. Balance a nuclear reaction by determining the type of nuclear process involved and the identities of all particles including mass and atomic numbers. |
Nuclear Reactions Overview |
||
| 1. Beta Decay |
2.
Electron
Capture |
3. Positron
Emission |
4.
Alpha
Decay |
| Answer these questions. 1. List the types of radioactivity and their identifying characteristics in order of increasing penetrating power. 2. What is "transmutation?" 3. What is ionizing radiation? 4. True/False a. Alpha decay decreases the mass of the nucleus b. Beta decay changes a nuclear neutron into a proton c. Electron capture changes a nuclear proton into a neutron d. Transmutation is a term used to describe one element changing into another e Alpha particles have greater penetrating power than gamma rays. 5. Identify each of the following decay processes and complete "X". 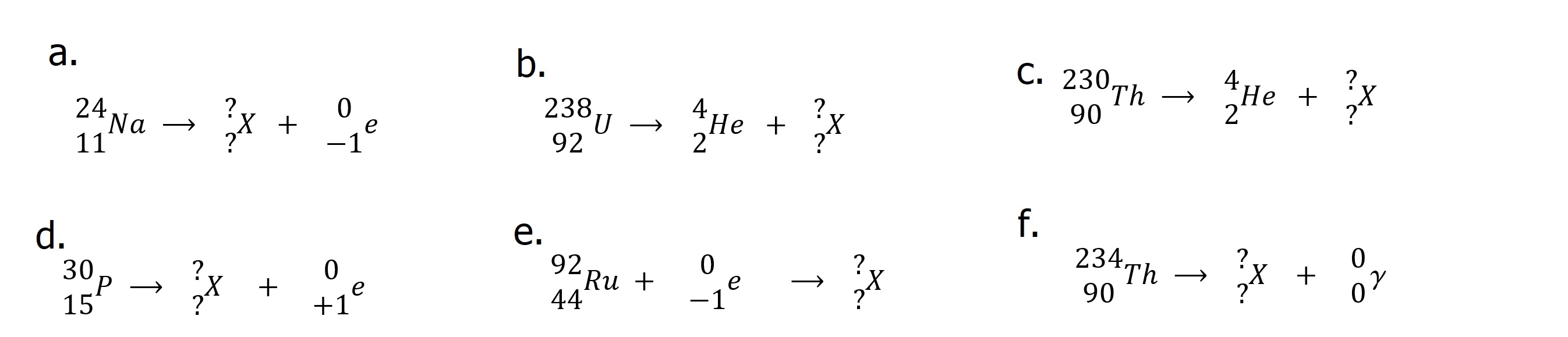 6. Identify the type of emission and the missing species in each of the following nuclear reactions. 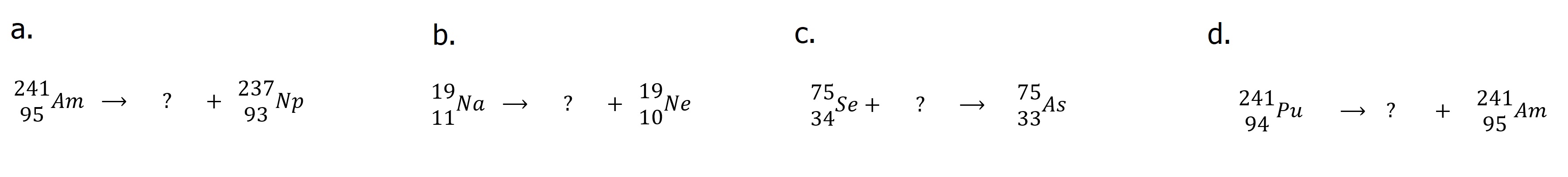 |
|||
| Answers: 1. Alpha particles ... helium nuclei....Lowest penetrating power Beta particles ... high speed electrons.... Medium penetrating power Positrons ... high speed positively charge particle (same mass as electron) ... Medium penetrating power 2. Transmutation is the result of nuclear processes which alter the proton count in a nucleus changing one element into another. Gamma Rays ... high energy electromagnetic radiation ... High penetrating power. Gamma Rays are often procuced in other decay processes. 3. Ionizing radiation (gamma and x-rays) has sufficient energy to ionize materials with subsequent damage especially to biological specimens. 4. a. True b. True c. True d. False 5. a. Beta decay 2412Mg b. Alpha decay 23490Th c. Alpha decay 22688Ra d. Positron emission 3014Si e. Electron capture 9243Tc f. Gamma ray emision 23490Th 6. a. Alpha decay 42He or 42α b. Positron emission 0+1e c. Electron capture 0-1e d. Beta decay 0-1e |
|||
Tuesday April 23, 2024 Day 69 Nuclear stability and predicting types of radioactivity |
|
| Course Lectures Please refer to the daily YouTube videos below |
Textbook References LT: 20.4: The Valley of Stability: Predicting the Type of Radioactivity OS: 21.1 Nuclear Structure and Stability |
Objectives 1. Determine whether an iosotope is stable or not based upon: a. the presense of even and odd numbers of protons and neutrons b. Magic Numbers c. Neutron/Proton ratio d. Position on the n vs p diagram. 2. Use the Band of Stability diagram to determine what type of nuclear decay takes place for an unstable nucleus. 3. Write balanced nuclear decay equations for unstable nuclei. |
Nuclear Stability and the Band of Stability |
| Answer
these questions.
Click and drag over the space below for answers. 1. Which of the following carbon isotopes is most stable and why? Carbon - 12 Carbon - 13 Carbon - 14 2. What is the N/P ratio for Tin - 100 and what type of nuclear decay will it undergo (if any)? 3. Why is manganese - 54 a stable isotope? 4. What are magic numbers and why are they important? 5. Why is Lead - 208 a stable isotope? 6. What type of decay most likely takes place for each of the following unstable nuclei? a. Polonioum - 210 b. Oxygen - 15 c. Radium - 228 7. Write nuclear equations that describe the decay of: a. Polonioum - 210 b. Oxygen - 15 c. Radium - 228 8. Write nuclear equations that describe the decay of: a. Phosphorus - 28 b. Uranium - 235 c. Calcium 37 d. Carbon-11 (electron capture) e. Thallium-207 |
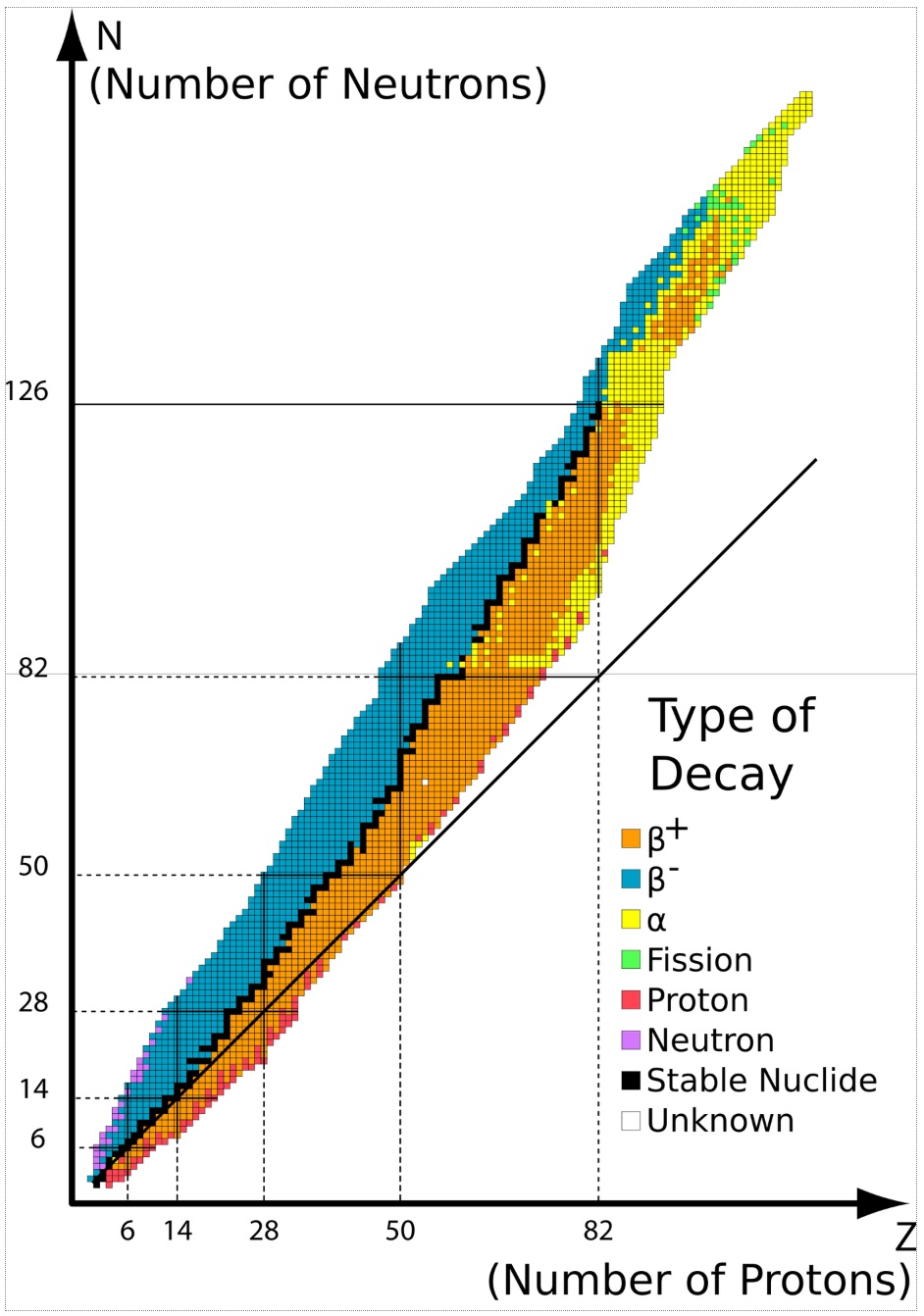 |
| 1. Carbon - 12 is most stable because there are even numbers of protons and neutrons and because the N/P ratio is 1:1. 2. Tin has 50 protons and (100 - 50) = 50 neutrons. This means the neutron to proton ratio is 1:1. Examining the figure above, this isotope falls to the right of the most stable isotopes (orange) making it likely to undergo positron emision. 3. Although there are odd numbers of protons and neutrons, the N/P ratio is 1.15 : 1 putting it in a stable region on the band of stability. 4. Magic numbers are experimentally observed preferred numbers of neutrons and protons in the nucleus. Note their positions in the diagram at right. For Protons: 2, 8, 20. 28. 50, 50 82 For Neutrons: 2, 8, 20, 40, 50, 82, 126 5. Pb- 208 has 82 protons and 126 neutrons. Both are magic numbers associated with stable nuclei and are therefore considered low energy and stable. 6. a. Atom is too massive. N/P ratio and figure above used to predict alpha decay 42α which lowers # protons, #neutrons and the mass of atom. b. N/P ratio and figure above used to predict positron emission 0+1e N/P ratio initially 0.875. After positron emission, N/P = 1.14 :) c. N/P ratio and figure above used to predict beta decay 0-1e N/P ratio initially 1.59. After beta deacy, N/P = 1.57 :) Closer to 1.5 7. a. 21084 Po → 42α + 20682 Pb b. 158 O → 0+1e + 157 N c. 22888 Ra → 0-1e + 22889 Ac 8. a. Initially, N/P ratio = 0.86 Need to increase neutrons and decrease protons to get N/P closes to 1. Positron emission increases n0 by one and decreases p+ by one. 2815 P → 0+1e + 2814 Si b. Initially N/P ratio = 1.55 Very heavy atom looking to shed mass via alpha decay . 23592 U → 42α + 23190 Th c. Initially N/P ratio = 0.85 Need to increase neutrons and decrease protons to get N/P closes to 1. Positron emission increases n0 by one and decreases p+ by one 3720 Ca → 0+1e + 3719 K d. Given electron capture, then we can write 116 C + 0-1e → 115 B e. Beta decay 20781 Tl → 0-1e + 20782Pb |
|
Wednesday April 24, 2024 Day 70 Radioactivity and 1st order decay |
|
| Course Lectures Please refer to the daily YouTube videos below |
Textbook References 20.5: Detecting Radioactivity 20.6: The Kinetics of Radioactive Decay and Radiometric Dating |
| Objectives: 1. Describe the meaning of half life and it's application to the first order decay processes. 2. Given a value for first order half life, calculate the decay constant λ 3. Use the first order integrated rate equation and the decay constant to calculate the amount of nuclear material remaining and consumed at any time for a nuclear decay. 4. Use C-14 measurements to predict approximate speciman ages. |
|
| Half Life Concept |
Nuclear decay calculations |
| Answer these questions.
Click and drag over the space below for answers. ec6629xv@go.minneapolis.edu 1. What type of reaction kinetics is displayed by nuclear decay? 2. Francium-223 has a half life of 22 minutes. What percent of a Francium-223 sample is left after... a. 22 minutes? b. 44 minutes? c. 66 minutes? d. 4 half lives? e. 10 half lives? f. 121 half lives? 3. Francium-223 has a half life of 22 minutes. What percent of an original sample is left after ... a. 22 minutes? b. 44 minutes? c. 66 minutes? d. 4 half lives? e. 10 half lives? f. 121 half lives? 4. Francium-223 has a half life of 22 minutes. How much of a 10.25 kg sample has decayed after 110 minutes? 5. Use the stated half life to determine the "decay constant.... λ" for each of the following radioactive elements (be sure to include correct units) a. Polonium-215 t1/2 = 0.00180 s b. Bismuth-212 t1/2 = 1.008 min c. Soidium-24 t1/2 = 15 hours d. Iodine-131 t1/2 =8.07 days e. Cobalt-60 t1/2 = 5.26 years f. Radium-226 t1/2 = 1600 years g. Urantium-238 t1/2 = 4.5 billion years 6. Use the 1st order integrated rate equation to determine how much of a 25.0 gram sample of Cobalt-60 is left after 12.5 months. The decay constant for Cobalt-60, determined above, is 0.13177 yr-1. 7. Technetium - 99 has a half life of 6.0 hours. How much of a 7.55 gram Tc sample has decayed after 1 week? 8. An ancient tree sample is found to contain 76.6 % of the original C-14 present when the tree was alive. Estimate the age of the tree sample. C-14 has a half-life of 5730 years. |
|
| Answers below: 1. 1st order reaction kinetics 2. a. 50 % b. 25 % c. 12.5 % d. 6.25 % e. 0.0977 % f. 3.76 x 10-35 % 3. a. 50% b. 75% c. 87.5% d. 93.25 % e. 99.9% f. 100.% 4. 9.92968 kg of the original sample has decayed. 5. a. 385.1 s-1 b. 0.68765 min-1 c. 0.0462 hr-1 d. 0.08589 day-1 e. 0.13177 yr-1 f. 4.33 x 10-4 yr-1 g. 1.54 x 10-10 yr--1 6. ln(Nt) = - λ t + ln(No) .... ln(Nt) = - (0.131yr-1) (12.5/12 yr) + ln(25.0) ..... ln(Nt) = 3.081608 ..... Nt = 21.8 8 grams remaining 7. λ = ln(2)/t1/2 = 0.11552 hr-1 ...... ln(Nt) = - λ t + ln(No) ....... ln(Nt) = 0.11552(168 hr) - ln(7.55) .... ln(Nt) = -17.3865 ... Nt = 2.812 x 10-8 g remains 7.55g - 2.812 x 10-8 g = 7.5499 grams have decayed. 8. λ = ln(2)/t1/2 = 1.209 x 10-4yr-1 ..... .. ln(Nt) = - λ t + ln(No) ....... t = [ln(No) - ln(Nt)] / λ .... t = [ln(100) - ln(76.6)] / (1.209 x 10-4yr-1) = 2203 years old |
|
Thursday April 25, 2024 Day 71 Nuclear Chemistry: Mass defect, Fission & Fusion |
|
|
|
|
| Course Lectures Please refer to the daily YouTube videos below |
Textbook References 20.7: The Discovery of Fission: The Atomic Bomb and Nuclear Power 20.8: Converting Mass to Energy: Mass Defect and Nuclear Binding Energy 20.9: Nuclear Fusion: The Power of the Sun |
| Chapter 19: Mass Defect |
Fusion and Fision |
| Answer these questions. Click and drag over the
space below for answers. Tutorial click HERE 1. What is meant by the "mass defect?" 2. Einstein's famous equation E = mc2 equates mass with energy. If c = 3.00 x 108 m/s, what mass units are required for energy to have units of Joules? 3. The Helium-4 nucleus has an actual mass of 6.6447 x 10-27 kg. Determine for this helium atom... (Source) a. the number of protons and neutrons. b. the mass of protons (1.6726 x 10-27 kg/p+) , neutrons (1.6749 x 10-27 kg/no) and the total mass determined by adding these contributions. c. determine the mass defect by subtracting the total mass you've just determined from the actual He-4 nuclear mass stated above. d. Convert the mass defect into Binding Energy using E = mc2 . Express your answer in Joules and eV. (1 eV = 1.602 x 10-19 Joules) e. To make Binding Energies compareable from one element to another, it is usually expressed on a "per nucleon" basis. Convert your answer in part "d" to eV/nucleon and MeV/nucleon (The number of nucleons is simply the number of protons and neutrons combined a.k.a. the "atomic number"). 4. Use the above steps to determine the mass defect and binding energy per nucleon for a nuetral iron-56 atom in units of MeV (Fe-56 nuclear mass = 9.2859 x 10-26 kg). 5. Use the above steps to determine the mass defect and binding energy per nucleon for a neutral fluorine-19 atom in units of MeV. (F-19 nuclear mass = 3.1548 x 10-26 kg) 6. Use the binding energies you've calculated above to put He-4, Fe-56 and F-19 in order of increasing nuclear stability. Question Answers below: 1. The mass defect is the mass difference between the actual mass of a nucleus and the mass obtained by adding individual proton and neutron masses. The mass defect represents the "binding energy" that holds the nucleus together which can be calculated via E= mc2. 2. kilograms (kg) NOT grams 3. a. 2 protons and 2 neutrons b. massp+ = 3.3452 x 10-27 kg massno = 3.3498 x 10-27 kg masstotal = 6.6950 x 10-27 kg c. 5.030 x 10-27 kg d. 4.527 x 10-12 Joules = 28,258,000 eV = 28.3 MeV e. 7.06 MeV/nucleon 4. 8.78 MeV 5. 7.52 MeV 6. Larger binding energies mean more stable nuclei. He-4 < F-19 < Fe-56 (Fe-56 is the most stable nucleus on a per nucleon basis) |
|
Friday April 26, 2024 Day 72 Medical Applications of Nuclear Chemistry |
||
| Course Lectures Please refer to the daily YouTube videos below |
Textbook References 20.11: The Effects of Radiation on Life 20.12: Radioactivity in Medicine and Other Applications |
|
| Ionizing
radiation and health |
How
Radiotherapy Works |
External
Beam Treatment: The Machine |
| Answer these questions.
Click and drag over the space below for answers. Activity: Use this calculator to determine your annual radiation exposure. How does it compare to the recommended 1 mSv/year recommendation for the public? 1. What is ionizing radiation? 2. How much radiation does the average person receive annually? Click on graphic at right for full EPA report? 3. What are the units used for radiation measurement and how are they related? 4. What are each of the following: i. Geiger counter ii. Dosimeter iii. Scintillation counter 5. What are internal and external radiation therapy? 6. What are direct and indirect cellular damage? 7. Why are cancer cells more susceptible to ionizing radiation and damage than healthy cells? 8. What is External Beam Treatment and how is the radiation controlled to provide maximum exposure to cancer cells while protecting healthy cells? 9. What are the four R's and how do they factor into cancer treatments? 10. Referring to video #3 (t = 3:37), How are X-rays produced in this radiation treatment? 11. Referring to video #3 (t = 5:30) How and why is the X-ray beam shaped in the device? |
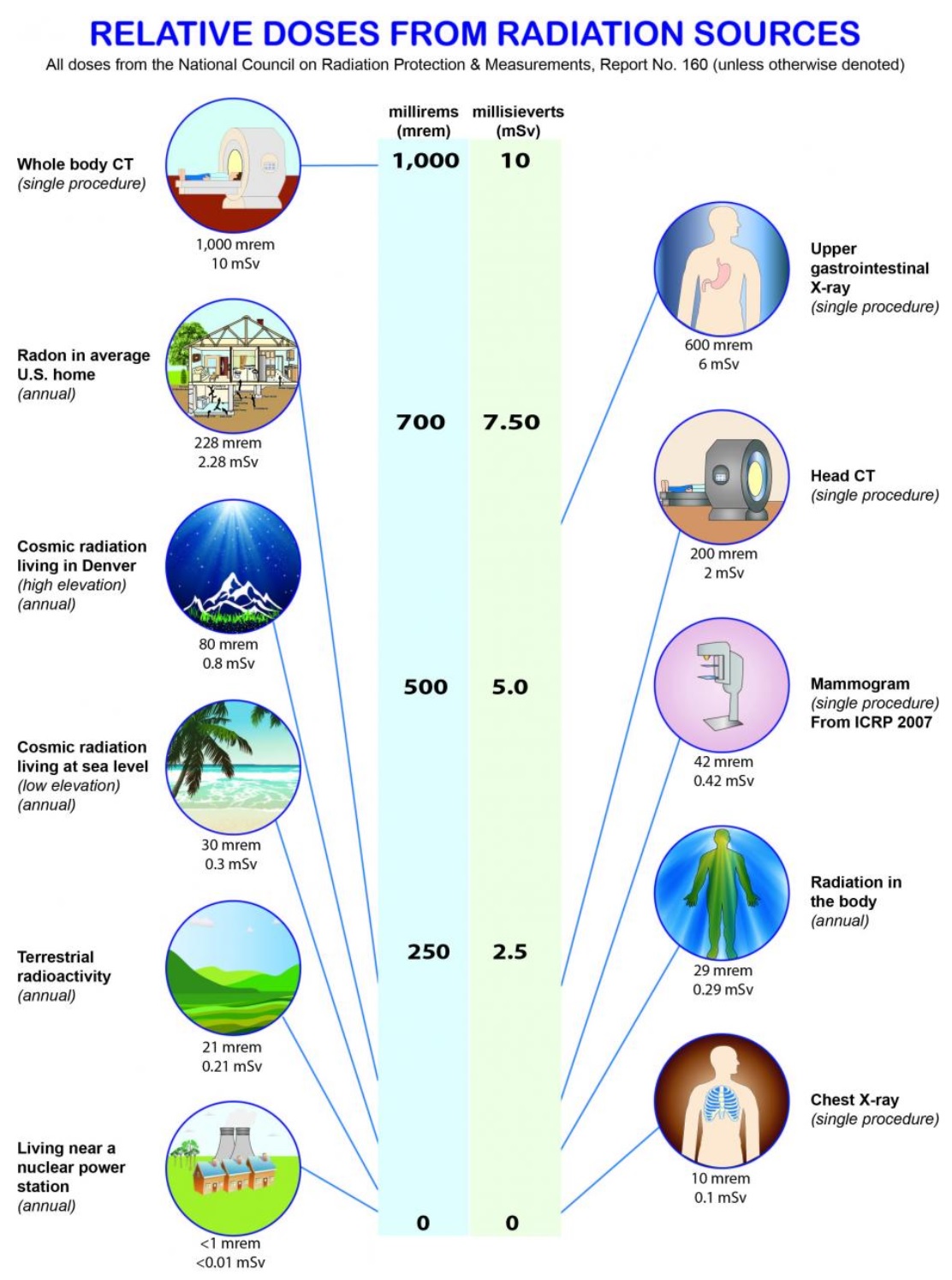 |
|
| Answers (Click and drag below): 1. radiation that strips a molecule of an electron leaving the molecule positively charged. 2. 6.2 millisieverts (mSv) 3. millirem (mrem) and millisievert (mSv) 100mrem = 1 mSv 4. . i: A device that signals an ionizing radiation as a "click" ii. A device that measure radiation exposure over time (a badge) iii. A device that signals ionizing radiation as a flash of light 5. Internal radiation therapy involves implanting small radioactive samples or "seeds" in cancerous tumors directly. External radiation therapy exposes cancerous tumors to external sources of radiation. 6. Direct cellular damage occurs when ionizing radiation acts directly on cancerous cells damaging their DNA Indirect cellular damage occurs as a result of ionizing radiation's interaction with water molecules producing free radicals and super-oxides which then damage the cancer cell's DNA 7. Cancerous cells reproduce more quickly than healthy cells and are therefore more likely to be in their vulnerable "M-Phase" of cell replication when radiation therapy is applied. Cancer cells are also believed to be less able to repair damage caused by radiation therapy. 8. Cancer treatment where the radiation source is external to the body. The radiation source is always kept pointed at the tumor providing maximum exposure.. However, by changing the position of the radiation source, nearby healthy cellular tissues receive a much small dose. 9. 1. Repair 2. Re-population 3. Reassortment 4. Re-oxygenation 10. High speed electrons created by the linear accelerator crash into a tungsten target producing X-rays. 10. Automated "fingers" absorb radiation and shape the beam. This shape or "beam profile" is adjusted to match the outline of the tumor continuously as the radiation source is moved around the patient. |
||