

| Monday April 22, 2024 Day 65 Vapor Pressure, Boiling and the Clausius-Clapeyron Equations |
|
Textbook Readings: 11.5: Vaporization and Vapor Pressure |
Course Lecture 11.3 pdf Video Vapor pressure and temperature 11.5 pdf Video Boiling |
| What is boiling? An Introduction |
Vapor Pressure and Boiling |
Objectives 1. Relate the ΔHvap to the relative strengths of intermolecular forces within the liquid. 2. Use the ΔHvap value to calculate the total heat required to convert liquid into gas at the boiling point temperature. 3. Describe how boiling bubbles are formed and what they're made of. 4. Use the Claussius Claperon equation to determine boiling point temperatures given pressure and ΔHvap values. |
Calculating Boiling Point Temperature Using Clausius Clapeyron Equation |
Homework Problems 73.1 Equal volumes of water and acetone are placed in 50 mL beakers. It's observed that the acetone disappears (evaporates) much faster. What does this observation tell you about how water's intermolecular (cohesive) forces compare to acetone's? 73.2 How does a water boiling bubble form and what gas is found inside? 73.3 Explain why liquids that experience hydrogen bonding have higher boiling point temperatures than other liquids. 73.4 ΔHvap has units of kJ/mol and tells us how much energy is required to vaporize 1 mol of the liquid. Use the following ΔHvap values to list the compounds in order of increasing boiling point temperature. Substance ΔHvap H2O 40.7 kJ/mol He 0.08 kJ/mol CH4 16.0 kJ/mol 73.5 A 50.0 mL water sample is heated to 100 oC. How much additional heat energy is required to completely vaporize the sample? Useful information for H2O: Density = 1.00 g/mL ΔHvap = 40.7 kJ/mol 73.6 How much heat energy is released when 1.50 grams of water vapor condenses? If the heat goes into warming a 75.0 gram iron block initially at 22.0 oC, what is the final temperature of the iron block? Useful information for H2O: Density = 1.00 g/mL ΔHvap = 40.7 kJ/mol Useful information for Fe : Specific Heat = 0.450 J/goC 73.7 Use the Clausius Clapeyron equations to determine the boiling point temperature of benzene at a pressure of 445 torr. Useful information for benzene: ΔHvap = 30.72 kJ/mol *Tboil = 80.1 oC at 1.00 atm pressure 73.8 Methylamine is a liquid that has vapor presssure of 344 torr at -25.0oC The liquid's normal boiling point temperature is -6.4 oC. Calculate ΔHvap for methylamine. * The boiling point temperature
that's given at 1 atm pressure is known
as the "normal boiling point" temperature. Click and drag the region below for correct answers 73.1 Acetone is a more volatile liquid, meaning it will evaporate MUCH faster than water. The reason for this is that acetone's intermolecular forces are much weaker than the hydrogen bonded water molecules making it much easier for acetone molecules to escape liquid's surface and become gas phase molecules. 73.2 At 100 oC, the vapor pressure of water is equal to or slightly greater than the atomospheric pressure. The vapor's pressure is now capable of pushing back the atmosphere and "blowing up" a boiling bubble. The bubble contains only water vapor. 73.3 Hydrogen bonding is a strong intermolecular force that keeps molecules from easily leaving the liquid to become a gas. Therefore, it takes more heat energy and higher temperatures to give the liquid phase molecules enough kinetic energy to escape. 73.4 It requires less heat energy to vaporize liquids with low ΔHvap values. Thus, these liquids will boil at lower temperatures. Consequently, the liquid that boils first will have the lowest ΔHvap value. Low boiling Point Temp..... He ..... CH4 ..... H2O ....... Highest boiling point temp. 73.5 113 kJ of heat energy is required to vaporize the water sample. 73.6 3.3897 kJ heat released. Fe block reaches a temperature of 122oC 73.7 Tb = 62.9oC @ 445 torr. Note that this result is consistent with the idea that boiling point temperatures are lower at lower atmospheric pressures. 73.8 ΔHvap = 23.45 kJ/mol |
|
| Tuesday April 23, 2024 Day 66 Sublimation and Fusion: Calculations |
|
Textbook Readings : 11.6: Sublimation and Fusion |
Course Lecture |
| Heat of Fusion and Heat of Vaporization Explained |
Sublimation And Deposition (Chemistry Demonstration) |
Objectives 1. Explain, on a molecular level, what happens for each of the following processes: i. sublimation ii. deposition iii. freezing (fusion) iv. melting 2. Write chemical equations describing each of the processes listed above (1). 3. Describe the temperature behavior during the fusion/melting process. 4. Use ΔHfus values to calculate the heat released/absorbed during the freezing/melting process. |
|
Homework Problems 74.1 Write chemical equations that describe i. ice melting ii. water freezing iii. ice subliming iv. water vapor depositing 74.2 When a solid melts or freezes, how does the temperature change. 74.3 As ice melts, the temperature stays constant at 0oC. What does additional heat energy accomplish if the ice temperature doesn't change? 74.4 How much heat energy is required to convert a 100.0 g ice cube at 0oC into liquid water at 0oC? For H2O ... ΔHfus = 6.02 kJ/mol 74.5 How much heat energy is required to sublime 50.0 grams of carbon dioxide solid at it's sublimation temperature? Useful information: CO2(s) ⟶ CO2(g) ΔHsub = 26.1 kJ/mol 74.6 How much heat energy is involved in depositing 50.0 grams of CO2(s) from the gas phase? Is this heat absorbed or released in the process? Click and drag the region below for correct answers 74.1 i. H2O(s) → H2O(l) ii. H2O(l) → H2O(s) iii. H2O(s) → H2O(g) iv. H2O(g) → H2O(s) 74.2 Okay, this is important. During the melting or freezing process, the temperature doesn't change. This is a bit strange since in the past we've always associated heat loss/gain with temperature changes. However, in the case of phase changes (freezing/melting or vaporizing/condensing), the heat lost or gained goes into making or breaking intermolecular forces; not into temperature changes. Short story: until a phase change has been completed, the temperature won't change. 74.3 If the ice is at 0oC, it is on the verge of melting. Additional heat energy doesn't increase the temperature of the ice. Rather, the heat energy breaks "hydrogen bonds" allowing the water molecules to break free from their crystal lattice locations. 74.4 33.4 kJ 74.5 29.7 kJ 74.6 This is exactly the reverse of the process in problem 74.5 (Note sign change on ΔH) : In other words CO2(g) ⟶ CO2(s) ΔHdep = -26.1 kJ/mol Thus, the calculated heat would be - 29.7 kJ corresponding to the release of this amount of heat energy. |
|
| Wednesday April 24, 2024 Day 67 Heating Curves and Calculations |
|
Textbook Reading 11.7: Heating Curve for Water |
Course Lectures 11.2 pdf Video Heat Calculations |
| Objectives 1. Calculate the heat absorbed or released at all points on a heating curve. 2. Determine the total heat lost or gained for a multi-step heating/cooling process. |
Heating Curves Tutorial: How to Calculate
enthalpy changes in Heating & Cooling* |
Homework Problems 75.1 Referring to the diagram at right: a. Calculate the heat energy required to increase the temperature of 50.0 grams of water from 20.00oC to 100.00oC. b. Calculate the heat energy required to vaporize 50.0 grams of water at 100.00oC to steam at 100.00oC? |
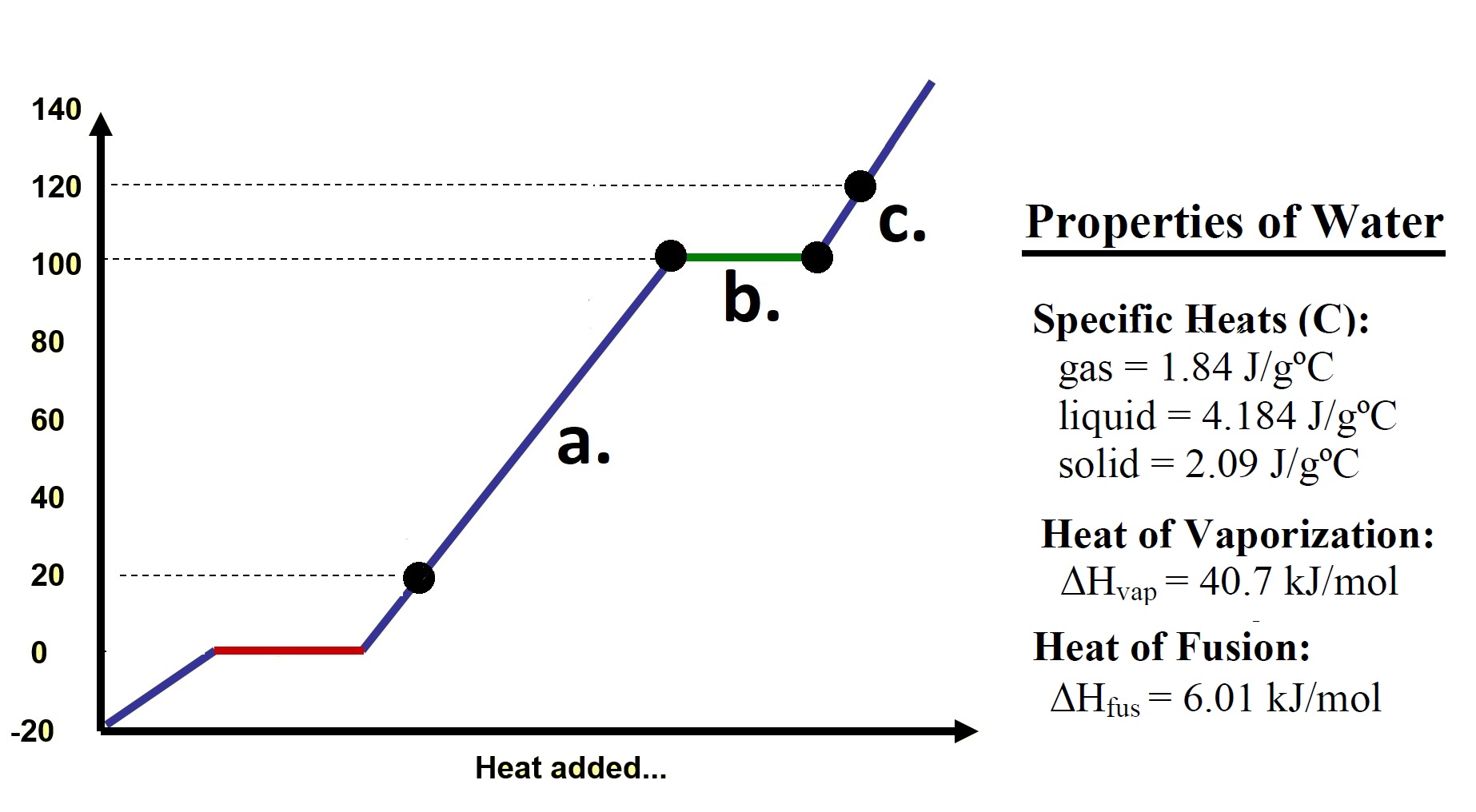 |
| c. Calculate the heat energy
required to increase the temperature of 50.0 grams of
steam from 100.00oC to 120.00oC? d. What is the total amount of heat required to convert 50 grams of water at 20.00oC into steam at 120.00oC? 75.2 How many joules (J) of heat energy are released when 6.80x103g of steam at 100.0°C is completely converted to solid ice at 0.0°C? Click and drag the region below for correct answers 75.1 a. q = m c Δ T = (50.0 g) (4.184 J/goC) (100.00oC - 20.00oC) = 16736 Joules b. q = n ΔHv = (50.0g/18.01g/mol) (40.7 kJ/mol) = 112.99 kJ c. q = m c Δ T = (50.0 g) (1.84 J/goC) (120.00oC - 100.00oC) = 1840. Joules d. 131.6 kJ 75.2 2.05 x 107 Joules |
|
| Thursday April 25, 2024 Day 68 Phase Diagrams |
|
Textbook Readings 11.8: Phase Diagrams |
Course Lectures 11.4 pdf Video Phase Diagrams |
| 11.4 Phase Diagrams |
Phase Changes, Heats of Fusion and
Vaporization, and Phase Diagrams |
Homework Problems Refer to the phase
diagram at right when
answering questions 76.1 - 76.6 76.1 What are the physcal states corresponding to regions i. , ii. , iii. and iv. 76.2 What is carbon dioxide's physical state at room temperature (~23oC) and atmospheric pressure (1 atm)? |
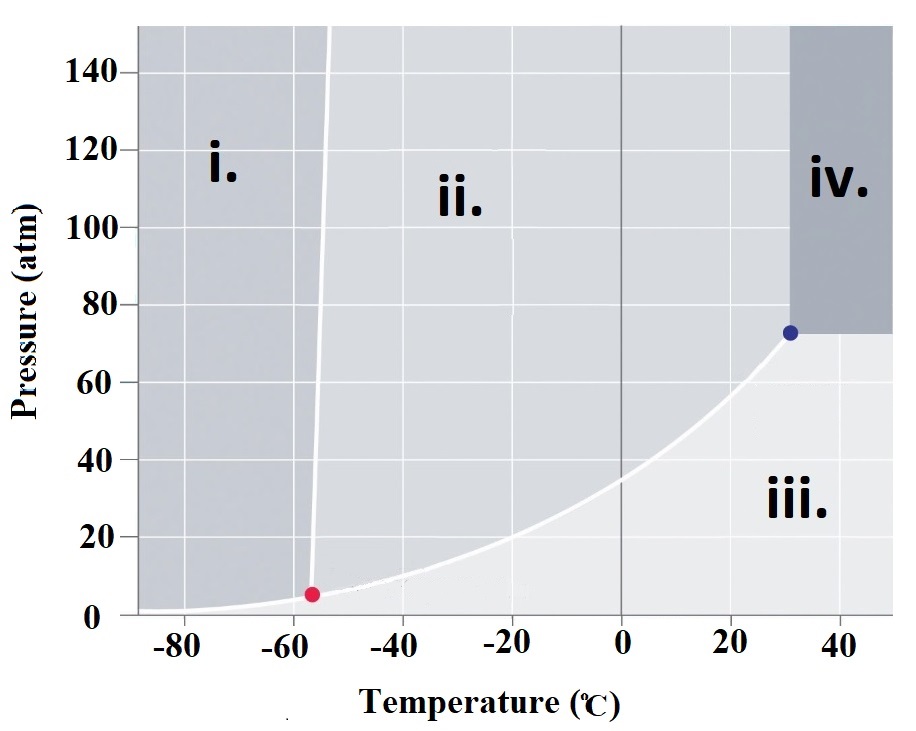 |
| 76.3 What it the meaning of the "triple
point" and what is the temperature and pressure corresponding to CO2's triple point? 76.4 What is the physcial state of CO2 at a pressure of 80 atm and a temperature of 40oC? 76.5 A CO2 sample at a pressure of 60 atm and a temperature of -20oC is warmed to a temperature of 40oC while keeping the pressure constant. What is the order of phase changes that occur along this path? 76.6 Consider a CO2 sample at 140 atm and -58oC. If the pressure is gradually decreased to 0.1 atm, what phase changes are observed? 76.7 What is a supercritical fluid? 76.8 Examine water's phase diagram available here. What phase changes occur as you go from point A to point B. What is so unusual about the order of these phase changes? Click and drag the region below for correct answers 76.1 i. Solid ii. Liquid iii. Gas iv. Supercritical fluid 76.2 Click here: Gas 76.3 Click here: The triple point is that set of conditions that CO2 can exist simultaneously as a solid, liquid and gas. For CO2, this corresponds to T = -56.57 oC and P = 5.11 atm. 76.4 Click here: Supercritical Fluid 76.5 Click here: Liquid -> gas 76.6. Click here: Decreasing the pressure eventually leads to the solid converting into a liquid. Finally, the liquid changes into a gas. . 76.7. A supercritical fluid exists at pressures and temperatures where liquid and gas phases become indistinguishable. View this video link for more information on supercritical fluids. 76.8 As pressure is increased, the density of the material must increase. This water system begins as a gas. As pressure is increased, the gas is converted into a solid. As even more pressure is applied, the solid is converted into a liquid. In other words, solid water (a.k.a. ice) is more dense than water vapor. However, ice is less dense than liquid water. For most materials, the solid phase is the most dense. However, in the case of water, the liquid phase is the most dense! |
|
| Friday April 26, 2024 Day 69 WATER! A Unique Substance! |
|
Textbook Readings 11.9: Water - An Extraordinary Substance |
Course Lectures 11.9 pdf Video Water; a unique substance |
| Objectives 1. Identify water's unique characteristics and give examples of each. |
Water - Liquid Awesome: Crash Course
Biology #2 |
Homework Problems 77.1 What's so special about water? 77.2 Water is a very polar solvent and therefore best dissolves polar solutes. Which of the following would not dissolve well in water? a. CO2 b. O2 c. K2SO4 d. CH3OH e. KCl f. HNO3 g. AgCl 77.3 The density of solid water (a.k.a. ice) is less than that of liquid water. Why is this and why is it important in nature? 77.4 Local large bodies of water help control the temperatures of areas around them. How is this possible? 77.5 Why do you feel cooler when sweat evaporates? 77.6 When diluting strong acids with water, the process is exothermic and generates a lot of heat. Consequently, the rule is to ALWAYS add the acid to water and NOT add water to strong acid. Why is this? (Specific heats: c H2O = 4.184 J/goC c H2SO4 = 1.34 J/goC Click and drag the region below for correct answers 77.1 Water molecules form strong hydrogen bonds Liquid water is very cohesive. Water is the only substance known to exist in all 3 forms (solid, liquid and gas) naturally on the surface of the earth. Water is a polar solvent and dissolves ionic substances easily. It is an "Amazing Solvent!" Water has a very high specific heat. 77.2 CO2 & O2 are non-polar molecules and don't dissolve well in water. AgCl is insoluble (think solubility rules) and doesn't dissolve well in water. 77.3 Unlike most substances, the density of solid water (a.k.a. ice) is LESS than the density of liquid water. Consequently ice floats. We observe this when as ice cubes floating in a drink. Also, forms and floats on the top of a lake while the majority of the lake is now insulated and stays liquid (Fish are very thankful!). The spacing between molecules for ice is GREATER than the spacing between molecules for liquid water. Because ice contains more empty space, it is less dense. 77.4 Water has a high heat capacity. This means it can absorb a lot of heat energy without significantly changing its temperature. With so much heat stored in the neighboring water, less is available to heat the surroundings. 77.5 As water evaporates (an endothermic process), hydrogen bonds are broken and this energy is removed. Removing energy lowers the temperature of the skin. This is especially significant because of water's high ΔHvap 77.6 Diluting acids produces a lot of heat and so to avoid possible (& dangerous) boil-overs, we want to minimize the temperature change that takes place. Water has an especially high specific heat in comparison to other liquids and this means it can absorb a lot of heat and only experience a modest change in temperature. So, adding acid to water let's the water absorb most of the heat and keeps the temperature under control. |
|